The exciting and confusing landscape of digitalisation opportunities for batch records
Whilst consultants fantasize about Pharma 4.0 and wonder when AI will replace humans, most pharmaceutical manufacturing teams rely on paper batch records, partially or entirely. For a reason: implementation of MES with integrated sensors and data is a costly and lengthy transformation process.
However, the drawbacks of paper-based processes are significant: repetitive basic checks, poor collaboration functionalities (post-its!), no possibility for simultaneous work, zero data for analysis, no reusable data for other documents.This leads to lengthy reviews, delayed error spotting, extended cycle times and bored teams who miss errors.
In 2026 however, digitalisation and automation are no longer an all-or-nothing, multi-million dollar question. It is a large, diverse landscape of solutions that bring different combinations of digitalisation, automation and required investments.
In this article we outline the different options and our own view on the investment/reward trade-offs.

How to Evaluate the Different Digital Batch Review Solutions
💡Key Evaluation Dimensions
Not all digital solutions address batch review challenges in the same way. To compare them effectively, pharmaceutical companies should evaluate each option across 8 critical dimensions:
1 - Overall Implementation Complexity & Costs:
- Organizational & integration impact: change requirements on current processes
- Total cost of ownership: licenses, validation, and ongoing maintenance
- Time to value: how quickly the solution delivers measurable efficiency gains
- Scalability across sites and products: ability to extend beyond initial scope
2 - Efficiency gains and overall impact on Quality and Teams:
- Impact on batch review speed: reduction in review time and release cycles
- Impact on quality: impact on speed of detection & resolution, deviation trends
- Gathering of process analytical data and manufacturing data
- Satisfaction at work: impact on team motivation and satisfaction
⚙️Complexity vs Impact Framework for Batch Record Reviews
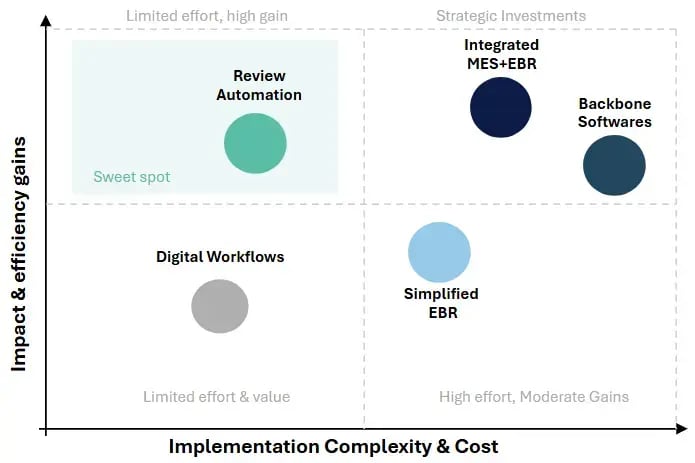
- Low complexity, low impact: Fast to deploy, limited automation value
- High complexity, moderate impact: High effort, incremental review improvements
- High complexity, high impact: Transformational but resource-intensive
- Low complexity, high impact: The "sweet spot" for fast, measurable ROI
💡Key Insight: Emerging New Solutions
Machine-learning-powered document automation delivers high impact with relatively low complexity by targeting batch review bottlenecks without disrupting existing MES or shop-floor operations.
Integrated Electronic Batch Records: The Gold Standard
What EBR Solutions Address
EBR systems replace paper batch records with structured, digital workflows at the shop-floor level.They guide operators through manufacturing processes in real time while capturing data electronically, improving execution accuracy and reducing transcription errors.
Implementation Reality
EBR deployments are powerful but require substantial commitment:
- Deep integration with MES, automation systems, and ERP platforms
- Extensive process redesign: workflows must be digitized and standardized
- Significant validation and training: operators and reviewers need new skills and procedures
- For full integration of data: retrofitting of machine with sensors, when possible
As a result, EBR projects deliver long-term value but require considerable upfront investment and multi-year implementation timelines.
✅Pros
- Eliminates paper at the source, improving data integrity
- Improves manufacturing accuracy and right-first-time execution
- Delivers strong long-term efficiency gains across the manufacturing lifecycle
⚠️Cons
- High upfront investment in software, integration, and validation
- Long deployment timelines, often 18–36 months for full sites
- Less suitable for organizations seeking fast, incremental improvements
Selection of leading EBR Vendors
- Werum PAS-X – Industry-leading MES with strong EBR capabilities
- MasterControl – Comprehensive eQMS & MES with strong document and training management
- SAP Digital Manufacturing – Integrated with SAP ecosystem for end-to-end visibility
- Siemens Opcenter Execution Pharma – Comprehensive manufacturing execution for pharma
- Emerson DeltaV / Syncade – Process automation with built-in batch management
- ETQ Reliance – Flexible quality management platform with manufacturing integration
- Leucine – Cloud-native EBR platform designed for agile deployment
Batch Record Review Automation: The Fast ROI Solution
What Makes This Category Different
Machine-learning-based batch record automation focuses on streamlining the review process without replacing existing manufacturing execution systems. This targeted approach addresses the primary bottleneck while enabling further digitalization steps, such as paper-on-glass or EBR adoption. These solutions typically:
- Ingest existing batch records (PDF, scanned images)
- Extract and validate critical data automatically using ML or LLMs
- Flag discrepancies, missing entries and deviations based on learned patterns
- Enable focus on exceptions instead of repetitive, page-by-page checks
Why ML Document Automation Delivers Faster ROI
Key advantages include:
- Minimal disruption to shop-floor operations
- No changes to manufacturing execution required
- Faster implementation compared to EBR or MES projects (weeks to months vs years)
- Significant reduction in manual review effort, typically 50%–60% time savings
- Improved consistency and audit readiness
- Standardized checks reduce variability and human error
This combination of high impact and lower complexity makes ML automation an attractive entry point for batch review digitalization.
Leading Solutions
- Acodis – Automation of document reviews and compliance checks, specialized in pharma batch records, quality and regulatory documents
- Aizon – AI-powered platform that optimizes pharmaceutical manufacturing by providing real-time, GxP-compliant predictive analytics
- Mareana – AI-powered platform for manufacturing intelligence, including workflow and document automation
- Ad-hoc – Deployment by agentic solution providers such as Tulip or ThinkTrends
✅Pros
- High impact on batch review speed with measurable time savings
- Lower implementation complexity than EBR or MES replacements
- Faster time to value, often delivering ROI within 6–12 months
- Scales well across multiple sites without heavy integration
⚠️Cons
- Does not replace MES functionality at the manufacturing execution layer
- Performance depends on document clarity
- Requires configuration and adaptations to company-specific rules
Digital Document Management System: The Workflow Support
Role in Batch Review
Document management systems provide a digital foundation by enabling centralized storage, version control, audit trails, and electronic review and approval workflows. They are often the first step in the digitalization journey, replacing physical archives with structured digital repositories.
Limitations for Batch Automation
While valuable for compliance and organization, document management systems do not fundamentally change how batch review is performed. Manual checks remain the dominant activity—reviewers still read through records page by page, just on a screen instead of paper.
Key Providers
- AmpleLogic – Purpose-built for regulated document control
- Informetric – Pharmaceutical-focused document and knowledge management
- Kivo – Quality document management for life sciences
- OpenText Documentum – Enterprise content management with life sciences capabilities
✅Pros
- Improved document control, searchability, and traceability
- Easier compliance management with centralized audit trails
- Relatively lower cost and faster deployment
⚠️Cons
- Limited automation intelligence or decision support
- Modest impact on batch release timelines
- Does not reduce reviewer workload significantly
Backbone Manufacturing and Quality Software: The Broader Transformation
What These Systems Do Well
Enterprise backbone platforms provide a comprehensive foundation for quality and manufacturing operations. They typically support:
- Electronic quality management (documents, deviations, CAPA, training)
- Integration with manufacturing and laboratory systems
- End-to-end traceability and audit readiness
These platforms are often central to long-term digital transformation strategies.
Trade-Offs
Despite their strengths, backbone systems come with significant challenges:
- Long implementation timelines, often spanning years
- High validation and change management effort
- Substantial license and integration costs
- Broad scope, even when batch review is the primary pain point
Selection of Top Providers
- Veeva
- MasterControl
- TrackWise (Honeywell)
- ETQ Reliance
- TetraScience (focus on data rather than documents)
✅Pros
- Strong compliance backbone
- Suitable for global, multi-site organisations
- Robust audit readiness
- Modularity: possibility to combine elements for more tailored solutions
⚠️Cons
- High cost and complexity
- Slow time to value for batch review improvements
- Often over-scoped for targeted automation initiatives
Side-by-Side Overview of Solution Categories
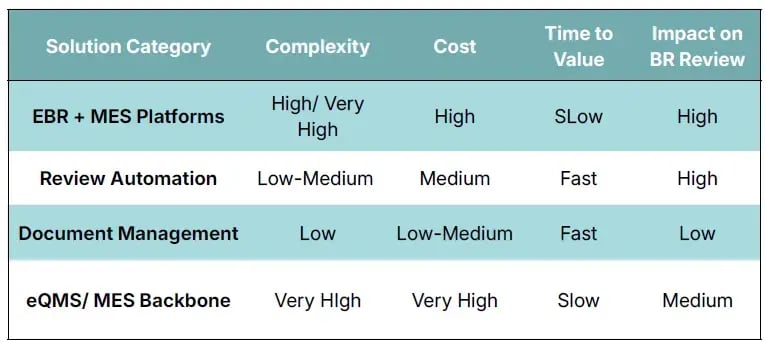
⚙️When Each Category Makes Sense
- Smaller or mid-sized organizations benefit most from ML-powered review automation as a starting point.
- Existing sites gain the fastest ROI by layering automation on top of existing processes.
- New facilities can justify full EBR and MES deployment from the ground up.
- Global enterprises often adopt a hybrid approach – EBR for strategic sites and ML automation for quick wins or selected processes.
The right choice depends on your organization's maturity, resources, timeline, and strategic priorities.
Recommended Digitalisation Roadmap for Pharma Companies
1. Automate Batch Review with ML Document Automation
Target the primary bottleneck first to achieve rapid efficiency gains with minimal disruption. Prove the value of digitalisation, structured data, and incremental change.
2. Improve overall process with digital workflows and integrations
With the possibilities of digital review, assess potential to optimise the whole review process for maximum efficiency, speed and quality. Build integration into other systems (QMS, SAP).
3. Expand into digital BR and EBR
Deploy Paper-on-Glass based on the digitalised MBR. Over time, connect MES and laboratory systems for complete digitalisation and automation.
Conclusion: Balancing Speed, Flexibility and Costs
There is no one-size-fits-all solution for digitalising batch record reviews. Each technology category comes with specific trade-offs between cost, complexity, and impact.
The key is to match technology choices to your organization's specific needs, maturity level, budget and strategic timeline. Software however is a space where incremental learnings and roll-out tend to be more rewarding than rigid all-or-nothing implementations.
If you are still reading this, we should talk: click here
